This page is part of the HL7 Europe Laboratory Report (v0.1.0: STU 1) based on FHIR (HL7® FHIR® Standard) R4. This is the current published version. For a full list of available versions, see the Directory of published versions
| Official URL: http://hl7.eu/fhir/laboratory/ImplementationGuide/hl7.fhir.eu.laboratory | Version: 0.1.0 | |||
| Active as of 2024-02-26 | Computable Name: Hl7EuLaboratoryIg | |||
Copyright/Legal: Used by permission of HL7 Europe, all rights reserved Creative Commons License |
||||
Obligations have been added to this version of the guide only as Informative material to collect feedback about their usage.
For more details about obligations please refer to the Obligations page
Clinical laboratory results play an important role in diagnosis, treatment, and follow-up of patients. The availability of high quality test results, and the capacity of sharing them, is therefore essential being often the basis for clinical decision making. For this reason the Laboratory has been selected as one of the priority domains for the European EHR eXchange Format (E-EHRxF).
Specify a set of rules to be applied to HL7 FHIR to define how to represent a Laboratory Report in the European Context, coherently with the European eHN Guidelines (see the European eHealth - Key documents ).
This Implementation Guide applies to laboratory reports within the core fields of in-vitro diagnostics, for example clinical biochemistry, haematology, immunohematology, microbiology, immunology, while leaving out some specialised laboratory domains like histopathology or medical genetics. This version focuses only on common rules that apply to all the in-scope situations, without specifying specialized domain-specific profiles, as for example microbiology profiles.
This guide is not limited to test results performed by clinical laboratories on Human specimens (from human subject), but it considers also results on non-human materials or living subjects; or non-human specimens paired with a human subject. Derived guides may restrict the scope as needed (e.g. limiting the scope to well-identified human beings)
The goal of this Implementation Guide is to define an European standard for the Laboratory Report to facilitate the harmonization among the national initiatives and prepare the ground for the European EHR eXchange Format (E-EHRxF).
This project is promoted by HL7 Europe, but realized in collaboration with several other European and national organizations and projects.
The aspiration of this guide is that of being used as basis for European National Guides, the European EHRxF and - consequently - by MyHealth@EU for the EU cross-border services.
The project background is described in the background page.
The solution adopted by this guide - and detailed in the Design choices page - balances the business requirement of Laboratory Report as legally signable document (i.e. as a FHIR document), with the expectation to get Lab Report by searching per DiagnosticReport. All this, taking into account the R5 DiagnosticReport design pattern where the DiagnosticReport <-> Composition relationship is directed from the DiagnosticReport to the Composition resource. This is done by supporting both perspectives (see figure below) requiring the document bundle ( BundleLabReportEu ) to always include a DiagnosticReport ( DiagnosticReportLabEu ) and enabling the pre-adoption of the R5 rules for the inclusion of entries in the Document Bundle.
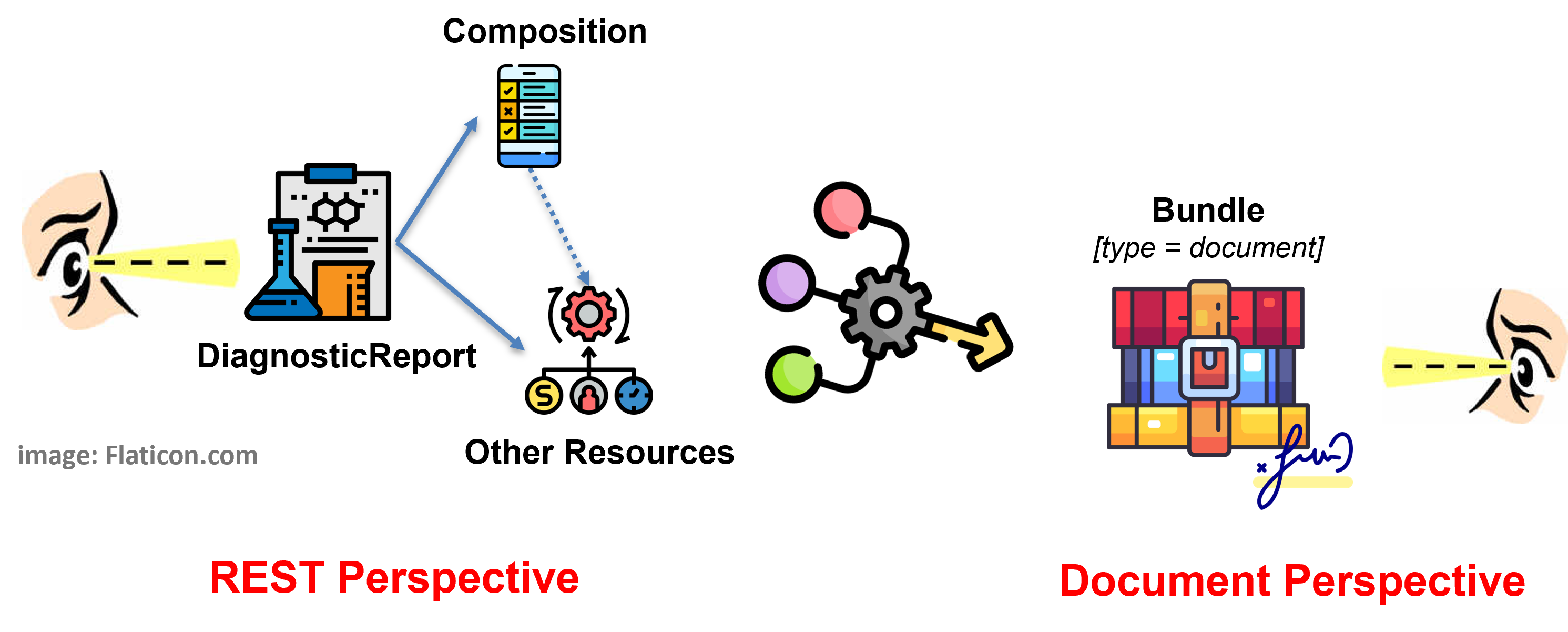
Figure 1 - Overview of the report design approach
The following diagrams provide a browseable overview of the profiles specified by this guide (not all the relationships have been reported).
The first highlights the most relevant relationships starting from the DiagnosticReport ( DiagnosticReportLabEu ) resource (REST Perspective).
Figure 2 - Overview of the profiles relationships
The second the profiles included in the document bundle ( BundleLabReportEu ) (Document Perspective).
Figure 3 - Overview of the document structure
| IG | Package | FHIR | Comment |
|---|---|---|---|
| hl7.fhir.eu.laboratory#0.1.0 | R4 | ||
| hl7.terminology.r4#5.3.0 | R4 | Automatically added as a dependency - all IGs depend on HL7 Terminology | |
| hl7.fhir.uv.extensions.r4#1.0.0 | R4 | Automatically added as a dependency - all IGs depend on the HL7 Extension Pack | |
| hl7.fhir.uv.ips#1.1.0 | R4 | ||
| fhir.dicom#2022.4.20221006 | R4 | ||
| hl7.fhir.eu.extensions#0.1.0 | R4 |
Package hl7.fhir.uv.extensions.r4#1.0.0 This IG defines the global extensions - the ones defined for everyone. These extensions are always in scope wherever FHIR is being used (built Sun, Mar 26, 2023 08:46+1100+11:00) |
Package hl7.fhir.uv.ips#1.1.0 International Patient Summary (IPS) FHIR Implementation Guide (built Tue, Oct 24, 2023 13:36+0000+00:00) |
Package hl7.fhir.eu.extensions#0.1.0 This guide lists the extensions speciifed for the European REALM. (built Mon, Feb 19, 2024 08:53+0100+01:00) |
This is an R4 IG. None of the features it uses are changed in R4B, so it can be used as is with R4B systems. Packages for both R4 (hl7.fhir.eu.laboratory.r4) and R4B (hl7.fhir.eu.laboratory.r4b) are available.
There are no Global profiles defined
This publication includes IP covered under the following statements.
Please refer to the Authors and Contributors page.