This page is part of the HL7 Europe Laboratory Report (v0.1.1: STU 1) based on FHIR (HL7® FHIR® Standard) R4. This is the current published version. For a full list of available versions, see the Directory of published versions
The European eHealth Network "GUIDELINE on the electronic exchange of health data under Cross-Border Directive 2011/24/EU Laboratory Results" - Release 1.1 is addressed to the Member States of the European Union and applies to the implementation of interoperable laboratory test result report cross-border exchange in order to support safe and efficient provisioning of care services in another member state.
It could also serve as a guiding principle for the national development and implementation of Laboratory Result Reports.
The eHN Laboratory Guideline in section 4 specifies a LABORATORY RESULTS DATASET, which is a simplified logical model of a laboratory report. Data set comprises of several basic parts as visualised in the diagram below.
Figure 1: Laboratory dataset model
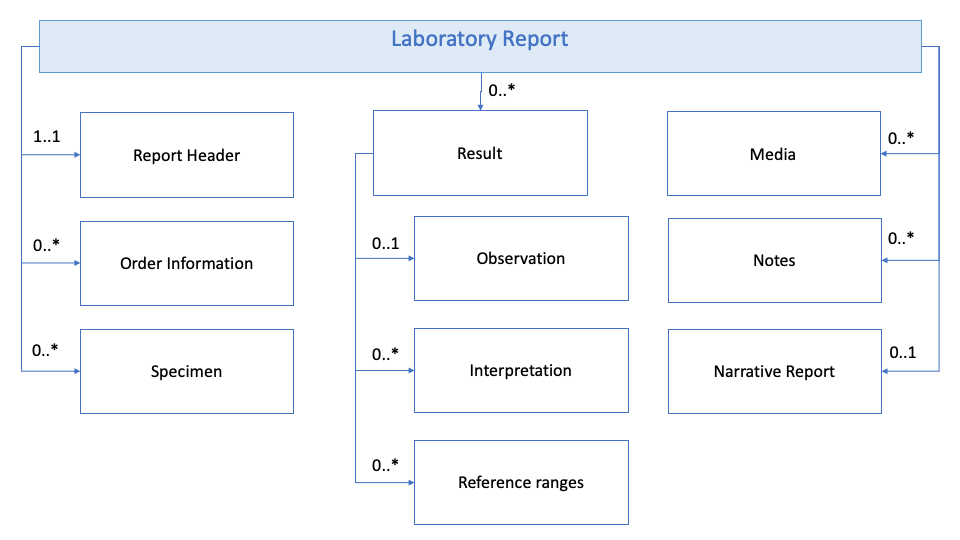
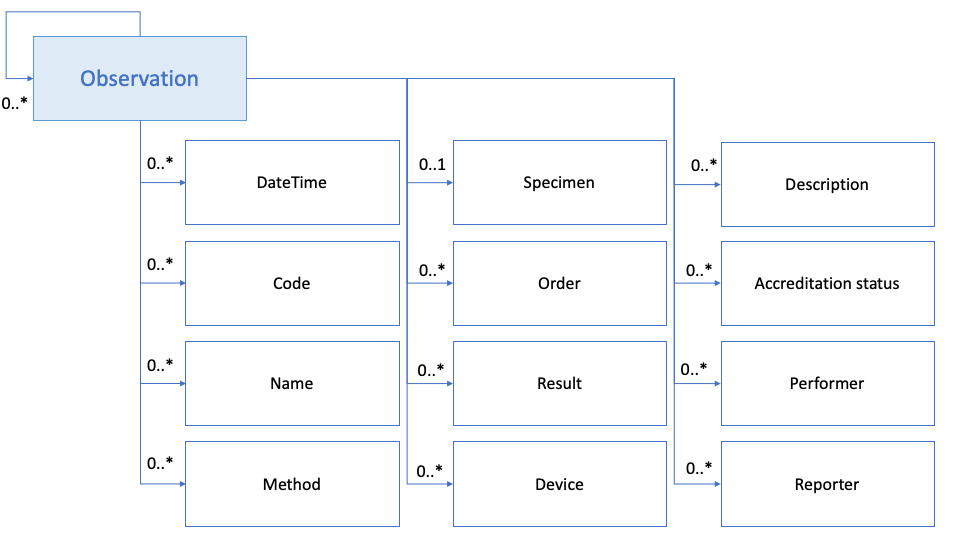
Figure 2: Laboratory observation dataset model
The following table lists the HL7 FHIR logical models used to represent the LABORATORY RESULT DATASET as defined in section 4 of the Release 1.1. of that eHN guideline.
To facilitate the references with the eHN data sets the short description of each element reports the label of the eHN element (e.g., A.1.7.2 Result validator name).
The HL7 FHIR logical model requires that element cardinality is specified, while the eHN data set doesn't define them on purpose. For this reason the elements' cardinality of the following FHIR Logical Model should be interpreted with this in mind, thus they should not be considered as "normative".
| Name | Title | Description |
| AuthorLabEhn | A.1.5 - Author | Author (by whom the Laboratory result report or a subset of its results was authored). Section A1.5 of the eHN guideline. |
| LabReportEhn | A - Laboratory Report | Laboratory Report. eHN guideline model. |
| LegalAuthenticatorLabEhn | A.1.6 - Legal authenticator | Legal authenticator (The person taking responsibility for the medical content of the document). Section A1.6 of the eHN guideline. |
| OrderLabEhn | A2, A3 - Order | Order information and reason. Sections A2 and A3 of the eHN guideline. |
| PayerLabEhn | A.1.3 - Health insurance and payment information | Health insurance and payment information. Section A1.3 of the eHN guideline. |
| RecipientLabEhn | A.1.4 - Information recipient | Information recipient (intended recipient or recipients of the report, additional recipients might be identified by the ordering party, e.g. GP, other specialist), if applicable. Section A1.4 of the eHN guideline. |
| ResultLabEhn | A.5 - Results data elements | Results data elements. Section A5 of the eHN guideline. |
| SpecimenLabEhn | A.4 - Specimen information | Specimen information. Section A4 of the eHN guideline. |
| SubjectLabEhn | A1.1, A1.2 - Subject of care | Patient or Subject of care. Sections A1.1 and A1.2 of the eHN guideline. |
| ValidatorLabEhn | A.1.7 - Result validator | Result validator. Section A1.7 of the eHN guideline. |